The Bends
In the vernacular of sport SCUBA diving, no two words conjure up more alarm, confusion, and debate. Since humans have taken their breathing gas with them, be it by surface supply or self-contained, decompression sickness (DCS) has plagued Homo aquaticus.
The name “bends” comes from the early observations of workers in the early 1800’s who spent considerable time working in pressurized mines or under riverbeds constructing foundations for bridges. These caissons, as the river work areas were called, were pressurized to keep the water out so the men could work. Since pressure effects on humans were largely unknown at this time, the workers would come straight to the surface after their 10 – 12 hour shift was over. Many of them would fall ill and some would be in such pain that they would double over barely being able to move about. This posture resembled a popular woman’s fashion at the time called the “Grecian Bend.” Essentially these women adopted a seriously bent forward stance when they walked (so much for the vanity of fashion). Although the official term was Caissons disease the moniker “bends” was attached to the men who became afflicted with the strange sickness. Many deaths were attributed to the bends well before it was studied or understood.
In 1878, Paul Bert published his theory that it was nitrogen bubbles causing Caisson disease. He cited the work of Robert Boyle who worked in the late 1600’s on reptiles with various pressure related experiments. Since these early observations, a tremendous amount of work has gone into trying to understand the effects of inert gasses within the body of a diver.
Words and Photos by Joe Dovala, Dovala Images
A gas is considered inert if it is not used in respiratory metabolism. Oxygen is not inert because it is metabolized, therefore it doesn’t get a chance to build up within the tissues. The respiratory waste gas carbon dioxide is very efficiently removed to the lungs and so is not a major contributor either but the nitrogen component (approximately 79% in air), which is not consumed during metabolism or removed efficiently by the body, is a big concern.
Tissue Compartments – Fast and Slow
While diving equipment and techniques have made very significant advances, some of the basic guiding principals of decompression table development have not really changed since the first postulations of John Scott Haldane, et al in 1907 and further refinements which culminated with A.A. Buhlman’s book in 1983. Most current recreational decompression tables and computers are based on neo-Haldane/Buhlman models of theoretical “tissue compartments.” These compartments are hypothetical cross sections of tissues that could be assigned numbers based on their ability to absorb and release inert gas based on half-times (the time at which a tissue is at 50% of its gas saturation).
There are “fast” tissues such as blood and “slow” tissues, such as bone. For example, a fast tissue might be at 50% saturation within 5 minutes where as a slow tissue might take hours to reach the same level. Obviously your body is not constructed of walled partitions, however, by conceptually arranging the body this way Haldane could come up with a mathematical model that could be manipulated and tested. In order to make some sense of gas loading, Haldane through experimentation arrived at a tissue nitrogen pressure ratio of 1.58:1. That is, as long as the tissue nitrogen pressure was no more than 1.58 times the ambient pressure, nitrogen would stay in solution in any particular tissue.
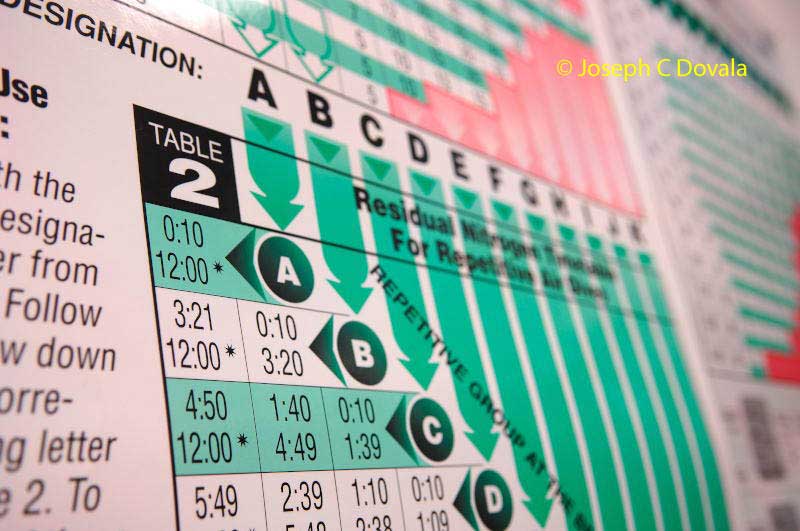
This proved to be only partially correct and much too simplified, as later experiments concluded that micro bubbles form much easier in supersaturated tissue than in Haldane’s experimental model of pure liquids. But it was a good starting point and was considerably better then anything else at the time. After much trial and error he devised a schedule that would allow some prediction of safe bottom times and depths. By avoiding, or at least, minimizing super saturation of inert gas within these various tissues, and limiting the ascent rate, a diver should be able to circumvent larger bubble formation that can cause physiological harm. Therefore, the neo-Haldane/Buhlman tables basically set predefined limits of gas absorption through M-Values (tissue compartment’s maximum allowable pressure called tissue tension – created by Workman/US Navy 1965) which if these ratios are exceeded, shallow decompression stops starting at ten feet are applied to vent the excess gas loading. With this theory it is presumed to bring the diver to as close to the surface as possible while keeping saturated tissue tension within the limits of the M-Values. Staying close to the surface allows for the maximum gradient between inspired inert gas and what exists in the saturated tissues. This concept, and minor variations of it, are the decompression algorithms in most dive computers today.
Deeper Stops
In the 1970’s Brian Hills of Australia developed a decompression method with deeper initial stops than the USN tables, believing the USN timetables allowed too much bubble growth. Further advantages of deep stops were discovered accidentally by Richard Pyle, an ichthyologist in Hawaii, while waiting for fish specimens he had collected to equalize their swim bladders during his ascent from very deep dives. Pyle realized that he felt much better after the long strenuous collection dives when he incorporated deep stops.
By the late 1980’s, a number of researchers were defining the “bubble gradient model” (BGM) and incorporating short deeper stops before continuing with the ascent. Currently the two most popular desktop decompression software programs are the open source code (no cost) Varying Permeability Model (VPM) and the commercially available Reduced Gradient Bubble Model (RGBM – see sidebar). Bubble physics, or the mechanical principles of bubble formation, is the driving force behind both the VPM and RGBM models.
Essentially the theory behind the BGM is to assign decompression schedules that attempt to keep as much inert gas in solution as possible, or at least keep the bubbles that form on ascent as small as possible. Thus eliminating more dissolved inert gas through the circulatory system rather then allowing expansion of microscopic seed nuclei in the tissues. In other words, the idea is to prevent gas phase growth rather than trying to deal with the bubble after it has formed. This is the fundamental difference between the BGM and neo-Haldane/Buhlman theories. Bubble models take into consideration both dissolved as well as the free gas postulated to exist within the body and try to minimize volume increases during ascent.
Micro Bubble Formation
So how does a micro bubble form and how big does it have to get before causing problems?
It appears that bubbles grow from “seeds,” which are microscopic gas pockets and cavities within the diver’s tissue that form the nuclei for growth into larger bubbles. While no one is sure how these seed nuclei are created, there is speculation that they form from mechanisms such as fluid movement and cavities within the tissues themselves. They are with us on every dive and most likely exist even while we are lounging around the pool prior to our first day of diving! During the late 1960’s and 1970’s, the Doppler ultrasonic bubble detector was tested on a large number of subjects, and it was found that a considerable amount of gas phase nitrogen (bubbles) had formed in many divers despite following very conservative ascent schedules. The more aggressive US Navy (USN) tables showed even higher bubble development. What surprised researchers the most was that despite substantial bubble formation many of the test divers felt no symptoms of DCS. However, Doppler meters can only detect moving bubbles within the outgoing venous blood flow and not stationary bubbles within the deeper tissues themselves. Once in the venous out flow, unless in excessive amounts, bubbles make there way to the lungs and are diffused out through the alveoli. While there is some correlation between quantity of bubbles and predisposition to DCS, it is more involved than a certain fixed volume of gas phase beyond which symptoms develop.
Skin Tension
The primary considerations when looking at whether or not a bubble will grow are largely due to skin tension, elasticity of its surrounding environment (in this case, how flexible the tissue is), and of course Boyles law. Skin tension refers to how permeable the surface of the bubble is as not all bubble skins are the same. Research has shown that biological surfactants, such as fatty acids, can alter the permeability of a bubble’s skin thus making it more resistant to collapsing. Conversely, the rigidity of the surrounding tissue can put limitations on a bubbles expansion.
As Boyles law states, if the internal pressure of the bubble is equal to the outside (tissue pressure or tension) then the bubble will not grow. If a pressure gradient exists with the bubble’s internal pressure, either higher or lower than the surrounding tissue, then there will be an outward or inward flow of gas across the surface of the bubble. However, due to the skin tension mentioned earlier, the pressure inside a small bubble is actually higher than in a large bubble. This is a major reason why you would want to keep the size of the bubbles as small as possible to aid in off gassing. By calculating a defined radius of a bubble, beyond which it will grow, you can establish a theoretical baseline of how much ambient pressure will be needed to keep it from growing. In other words, by limiting bubble growth through deeper stops during ascent, dissolved gas will have a tougher time becoming free gas to produce larger bubbles. Further definition of this aspect of bubble physics would need a significant amount of mathematics to illustrate this concept. I, for one, usually find something else to do in a big hurry when row after row of numbers and Greek symbols make their appearance. So instead I’ll try a couple of simple metaphors (risking over-simplification), and leave web sites below this article for those with a more numerical appetite.
I can remember being very young and devising a plan to scare my mother by blowing up one of my birthday balloons, then popping it after sneaking up on her. The joke was on me though for I just ended up with a very sore jaw, because of how much effort it took to start the inflation on a fresh balloon. There are a number of factors for why a balloon gets easier to inflate as it gets bigger but it is the skin tension that concerns us. The skin tension of the surface of the balloon, like a bubble (ignoring other issues like surfactants for this example), varies inversely with the radius of the balloon. In other words, as the radius increases the skin tension decreases and the pressure inside is less than an identical smaller balloon. Eventually if the capacity of the balloon is exceeded it will pop. Therefore, to get outward diffusion working for us, we need to keep the pressure inside of micro bubbles as high as possible and keeping them small is a key strategy to accomplish this.
Observations On Bubble Growth
We can also observe the classic bottle of beer and make some observations regarding bubble growth. Before it is opened, the carbon dioxide (CO2) exists in equilibrium as dissolved gas within the beer. This gas is under tension as far more of it is dissolved than would be possible under normal ambient conditions (like that of your favorite watering hole). As long as the ambient pressure is such that micro bubbles can be kept from expanding in radius beyond a critical dimension, then there is no bubble growth. However, when the bottle is opened, bubbles quickly form at nucleation sites, such as scratches and imperfections on the glass surface. The bubbles coalesce and form a head of foam at the top of the liquid. Even a cursory look will show that many of the bubbles grow several times in diameter on their way to the top of the glass. Boyle’s law by itself cannot explain this, as there is not enough change in hydrostatic pressure from the bottom to the top of the bottle. It is the diffusion of dissolved CO2 from the liquid that penetrates into the forming bubble, allowing it to expand, which further reduces the pressure inside thereby allowing even more CO2 to enter, until it either joins the foam or equilibrium has been reached with the dissolved CO2.
Keep in mind that bubbles inside a diver do not only contain one type of gas. Besides the trace gasses found in air, there will be a varying amount of carbon dioxide, nitrogen, and oxygen depending on the mix the diver is using. So reducing the inert gas pressure within a bubble also depends on the gas gradient. In other words, the less molecules of an inert gas coming into the surrounding tissue, the less there will be to add to what is already inside the bubble.
For example, reducing the nitrogen pressure within a diver’s tissues not only is controlled by slow diffusion as in decompressing slowly, but also through “opening the oxygen window” and creating as large as discrepancy as possible between what is coming into the body and what is already in the tissues by inspiring high oxygen/lower nitrogen mixes during ascent. The less nitrogen going in the less that will be absorbed by the tissues. This decompression technique is the basis behind many technical diving courses and why enriched air nitrox gives you more bottom time compared to air.
Minimizing Risk
Another important point is to remember that decompression schedules are designed to minimize the risk of DCS. None can remove the risk entirely. All tables, computers, and chat line debates are strictly theory. Besides the physics involved in gas theory, there is a bewildering complexity of how inert gas behaves within a living system. There are thousands of biochemical reactions cascading through every organ and tissue with in your body at any given time. Many of these can directly or indirectly influence gas diffusion, micronuclei development, or even how a particular bubble will affect the tissue around it. There are many additional predisposing factors that can also contribute to becoming susceptible to DCS that can change from one day to the next from one individual to the next. These factors include hydration levels, amount of rest, workload, water temperature; the list goes on and on. Attempting to incorporate all these components is daunting to say the least and may never occur. However, despite the enormity of the challenge, as new research continues and there is active debate with real world testing, we will be able to continually improve our decompression schedules both in terms of safety and efficiency.
• • •
Sidebar
The VPM came out of work by the University of Hawaii (UOH) in the mid 1980’s on gelatin. In the late 1980’s, David Yount and Don Hoffman used the UOH findings to calculate an actual deep stop decompression table for air based on experiments with gelatin, shrimp, and salmon fingerlings. The table was only for air and extremely complex. In the mid to late 1990’s, Yount, Maiken, and Baker implemented the original algorithm with some changes including multiple gas switching, repetitive dives, and made the source code open for critical examination of assumptions and methods among tech divers in the field.
The RGBM model is very similar to the VPM as they share many of the same assumptions and methods. In the early 1990’s, Bruce Wienke explored resetting gradient parameters to extend the model to repetitive multi-day deep diving. The RGBM is the primary bubble model implemented in advanced diving computers and commercial deco software. The National Association of Underwater Instructors (NAUI) is the first recreational agency to recommend deep stops for recreational “no decompression” diving (see A Tenent Of Diving Fine-Tunned; DT June ’03)
Despite the zeal with which BGM decompression methods are debated and discussed among technical divers, their direct impact on recreational diving deco procedures will probably be minimal. These schedules are designed for extreme depths and exposures and can shave considerable hang time off of these planned decompression dives with increased safety. The “standard” no stop relatively shallow recreational diving most of us do are served quite well by the current schedules and practices of the responsible sport diver. Nonetheless, any innovative techniques to deal with the complexities of inert gas physiology will have long-term effects on improving the quality of our undersea explorations. After all, just a few years ago, Nitrox was considered by many industry “experts” as too risky and complicated for sport divers to use and should be kept in the realm of commercial diving only. It is now one of the most sought after specialties with the recreational dive training agencies.
Below are some websites for a more detailed look at the BGM:
http://www.decompression.org/maiken/VPM/VPM_Program_Site_Map.htm
http://home.hetnet.nl/~deep_ocean/index.html
http://website.lineone.net/~britannic98/prep/rgbm.htm
http://www.rgbmdiving.com/site/rgbmhis.htm
Words and Photos by Joe Dovala, Dovala Images